From Excel lists to digital test equipment management - why ISO 17025 demands more
Many testing and calibration laboratories still work with Excel lists or paper-based systems to organize their test equipment management. At first glance, this seems pragmatic: spreadsheets are quick to create, easy to adapt and already established in many organizations.
However, in the long term, this approach collides with the requirements of DIN EN ISO 17025 and DIN ISO IEC 17025. The limits become apparent at the latest when the number of measuring devices increases or several locations are involved. In addition, the limits become apparent at the latest when the number of measuring devices increases or several locations are involved. The problem is exacerbated by a generational change in auditors: younger auditors interpret ISO 17025 more precisely and more consistently. Laboratories should prepare for increasingly stringent audits - Excel no longer meets these requirements.
The consequences range from a lack of traceability and unclear calibration intervals to serious risks for audit compliance and accreditation. This is precisely where modern solutions such as specialized test equipment management software - for example laboratory software such as LabThunder- come in.
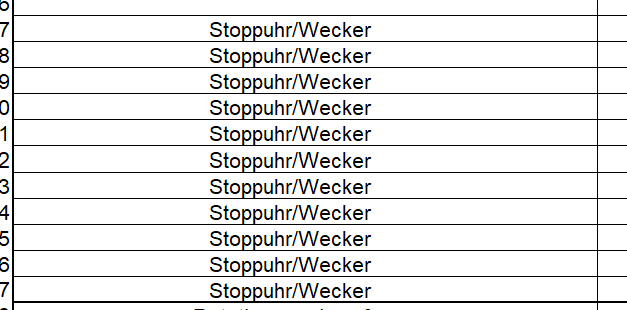
In this list excerpt, the test equipment designations are not individualized (e.g. only "stopwatch" several times). This leads to confusion and makes clear identification more difficult. A consistent, consecutive and unique designation such as "stopwatch 1", "stopwatch 2", "stopwatch 3" is recommended. This helps to avoid confusion and avoids the need for explanations during audits.
Standard context: What DIN EN ISO/IEC 17025 specifically requires
ISO 17025 regulates the competence of testing and calibration laboratories. Three sections are particularly relevant for test equipment management, measurement equipment management and measurement equipment monitoring:
§ 6.4 - Equipment
Laboratories must ensure that all test and measuring equipment is suitable for the intended activities. Each piece of test equipment requires unique identification, including assignment to calibration and maintenance data. In addition, records of calibration, adjustment, maintenance, repair and condition must be kept without gaps.
§ 6.6 - Externally provided products and services
If calibrations are outsourced, laboratories must ensure that the service provider meets the requirements - for example through accreditation. The traceability of the standards and calibration chains used must be documented.
§ 7.11 - Data and information management
Collected measurement data and metadata must be protected against loss, manipulation and unauthorized changes. Changes to data must be traceable - through change tracking and a complete audit trail. This also expressly applies to electronically managed records such as Excel lists, Access databases or other individual systems.
Standard-compliant test equipment management is therefore more than just an inventory list. It is about structured test equipment monitoring and a robust system for ensuring audit compliance.
Duties at a glance: What a laboratory must do organizationally
An ISO 17025-compliant system for test equipment management and measuring equipment management must cover the following areas:
Clear identification
Each piece of test equipment is given a unique test equipment number. There must be no risk of confusion due to duplicate or obsolete numbers.
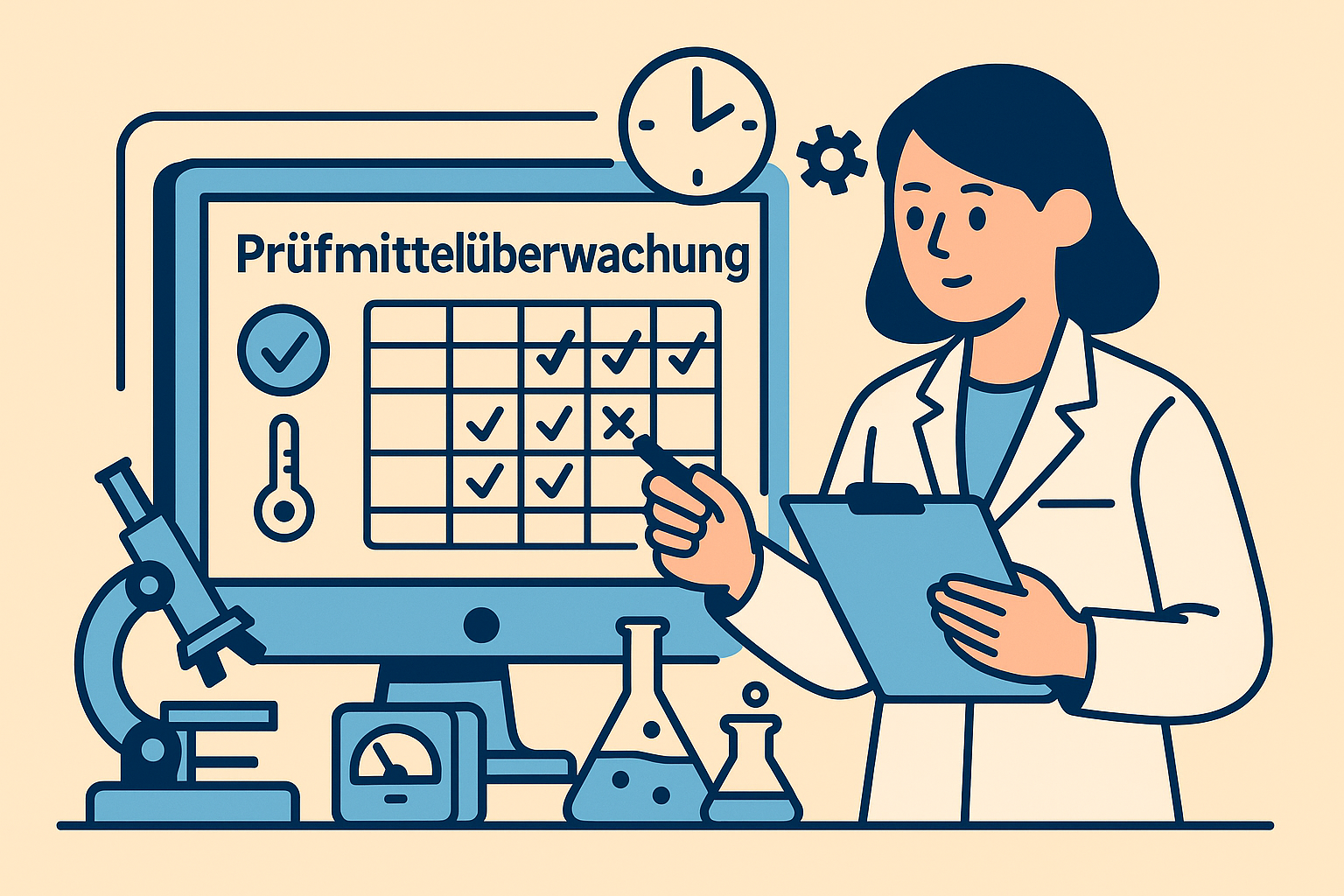
Calibration and measuring equipment monitoring
Calibration intervals must be defined and documented - based on risk, frequency of use and manufacturer specifications. Measuring equipment monitoring requires clear due dates, reminders and blocking logic for overdue calibrations. Calibration certificates and test certificates must be assigned and retrievable.
Traceability
Traceability to national or international standards must be ensured. The standards used, calibration laboratories and relevant characteristic values must be documented.
Maintenance and servicing
Preventive maintenance and repairs must be planned and documented. The status of test equipment - for example in the event of defects or blockages - must be clearly linked.
Status labeling
Each piece of test or measuring equipment must clearly show its status: calibrated/released, locked/out of service or in calibration/maintenance.
Theoretically, these requirements can be mapped in Excel - in practice, many laboratories fail to implement them consistently and error-free.
Audit reality: Typical errors in practice
Similar problems recur in audits according to DIN EN ISO 17025 or DIN ISO IEC 17025:
- Duplicate test equipment numbers in different Excel files or versions
- Missing or incorrect calibration intervals because changes were discussed but not updated in the table
- Calibration certificates that cannot be found locally or in e-mail inboxes
- Obsolete stocks: Test equipment that has long since been discarded still appears in the list
- Non-transparent changes to Excel files: No one can trace who edited which data and when
- Overdue calibrations because no automatic test equipment monitoring exists
Such deviations not only jeopardize audit compliance, but in the worst case also the validity of test and measurement results.
Why Excel fails in the long term
Excel was not developed for standard-critical test equipment management. The weaknesses are evident in four key areas:
1. data integrity
Files can be accidentally overwritten, corrupted or saved locally. Versions in different departments drift apart. There is no integrated logic to reliably enforce mandatory fields, plausibilities or blocking rules.
2. change tracking
There is no real audit trail. Changes can only be traced with considerable additional effort. However, ISO 17025 requires data changes to be documented and traceable.
3. calibration deadlines and reminders
Due dates must be maintained and monitored manually. As the number of test equipment increases, the list becomes confusing. Escalation mechanisms such as automatic blocking of overdue measuring equipment are missing.
4. multi-site and multi-user capability
Parallel access to an Excel file quickly leads to conflicts or inconsistencies. Different teams - such as testing, calibration and QM departments - do not have a common, reliable data status.
Excel can help temporarily, but digital test equipment management as defined by ISO 17025 cannot be implemented in a sustainable, secure and audit-proof manner.

Digitalization as a solution: what test equipment management software can do
Modern test equipment management software or test equipment monitoring software addresses precisely these weak points and creates a central, standard-compliant database.
Automated test equipment monitoring
Central master data for all test and measuring equipment enables automatic calculation of calibration deadlines and notifications when due. Status changes - such as blocking in the event of overdue items - are rule-based.
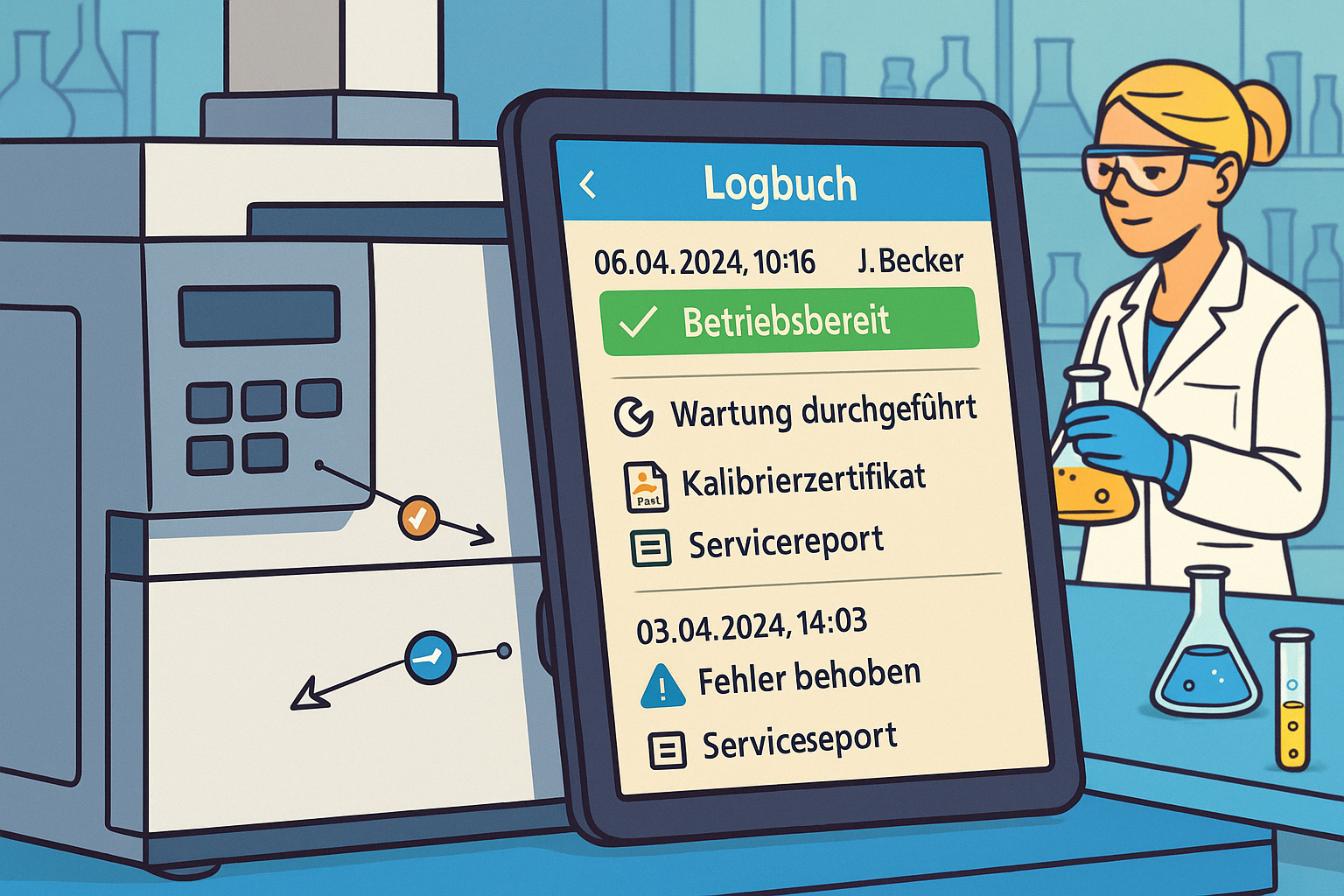
Complete audit trail
Every change to test equipment master data, calibration or maintenance information is logged. Responsibilities are clearly traceable - a key aspect of audit compliance.
Integrated document management
Calibration certificates and certificates are stored directly on the test equipment. This enables quick access during audits instead of time-consuming searches in folder structures or emails.
Standardized measuring equipment management
A standardized structure for test equipment and measuring devices across all laboratories and locations, combined with clear roles and rights for laboratory management, QM, technology and purchasing.
Integrated laboratory software combines test equipment management, test equipment monitoring and other laboratory processes such as maintenance planning, logbook and documentation. This creates a consistent, digital image of the laboratory reality - the basis for efficient processes and reliable accreditations.
Practical example: From Excel chaos to ISO 17025 conformity
A medium-sized testing and calibration laboratory with around 400 pieces of active test equipment had been working with several Excel lists for years. Each department maintained its own measuring equipment management, sometimes in different formats.
A surveillance audit revealed the following problems:
- Several test equipment with overdue calibration because due dates had been adjusted in an older Excel version but not adopted
- A calibration certificate for a critical reference standard could not be found
- Two devices had the same test equipment number - one in the main laboratory, one in a branch office
The result: deviations in the audit, extensive rework and increased pressure on QM and laboratory management.
The laboratory decided to switch to test equipment monitoring software and implemented LabThunder as a central platform:
- All test equipment was migrated in a structured manner and assigned unique IDs
- Calibration intervals, responsible persons and service providers are stored centrally
- LabThunder took over calibration monitoring with automatic reminders
- Calibration certificates were stored as PDFs directly on the respective test equipment
- The integrated audit trail has been logging every change ever since
In the next monitoring audit, the laboratory was able to prove at the touch of a button which test equipment is used for which procedures, when it was last calibrated and that all relevant documents are available in full. The auditors particularly emphasized the clear traceability and consistent test equipment management.
Conclusion: Without digital test equipment management, ISO 17025 becomes a risk
Anyone who takes the requirements of DIN EN ISO 17025 or DIN ISO IEC 17025 seriously will not be able to avoid systematic, digital test equipment management in the long term. Excel lists and manual workarounds may work in the short term, but they fail:
- Data integrity and traceability
- Continuous test equipment monitoring
- Transparent change tracking
- Multi-location and multi-user capability
Specialized test equipment management software ensures that calibration, maintenance, traceability and status marking are implemented in a standard-compliant and audit-proof manner - and relieves the burden on laboratory management and QM in the long term.
Audit-compliant, digital test equipment management with LabThunder
If you want to make your test equipment management, measuring equipment management and test equipment monitoring future-proof and ISO 17025-compliant, now is the right time to switch to a digital solution.
LabThunder offers you:
- Central, digital test equipment management for all devices and measuring equipment
- Automated calibration and maintenance reminders
- Complete audit trail and secure traceability
- Integrated laboratory software for logbook, maintenance, knowledge management and more
Find out more about how LabThunder can help your laboratory comply with ISO 17025 and digitize your processes sustainably.
Frequently asked questions about ISO 17025 and test equipment management
Is Excel permitted for ISO 17025 test equipment management?
Theoretically yes, but practically unsuitable. Excel can meet the formal requirements of the standard, but usually fails in terms of data integrity, change tracking, and multi-user capability. Modern auditors are becoming increasingly critical of Excel solutions because they lack a true audit trail and version conflicts are difficult to trace. Excel lists reach their limits when the number of test equipment increases or there are multiple locations.
What does ISO 17025 require for test equipment management?
ISO 17025 requires clear identification of all test equipment, complete documentation of calibrations and maintenance as well as traceability to national standards. In addition, a complete audit trail must be available for all data changes. The relevant clauses are § 6.4 (Equipment), § 6.6 (Externally provided services) and § 7.11 (Data and information management).
What is the difference between test equipment and measuring equipment?
Test equipment is used for qualitative assessment, such as gauges, test devices or test templates. Measuring equipment provides quantitative measurement values, such as scales, calipers or thermometers. ISO 17025 treats both under the term "equipment" and places the same requirements on identification, calibration and documentation.
What happens if calibration is overdue in the audit?
Overdue calibrations are considered major non-conformities in the audit. If measurements or tests have been carried out with overdue test equipment, in the worst case the accreditation for the affected procedures may be withdrawn. The laboratory must re-evaluate all affected measurement results and inform customers if necessary. Systematic overdue results indicate deficiencies in the management system.
How often must test equipment be calibrated in accordance with ISO 17025?
ISO 17025 does not prescribe any fixed calibration intervals. Laboratories must determine the intervals on a risk-based basis - depending on the frequency of use, manufacturer specifications, historical drift behavior and significance for the measurement results. It is important that the determination is documented and justified. Shorter intervals may be required for critical measuring equipment.
Can I lose my accreditation due to test equipment deficiencies?
Yes, this is possible. Serious deficiencies such as a lack of traceability, systematically overdue calibrations or a missing audit trail can lead to the suspension or withdrawal of accreditation. It becomes particularly critical if it is proven that faulty test equipment has led to incorrect measurement results. In such cases, laboratories must provide evidence of extensive corrective measures and, if necessary, inform customers.
What is an audit trail in test equipment management?
ISO/IEC 17025 does not explicitly require an "audit trail." However, in section 7.11 (Control of data and information management), the standard requires that laboratory and test data be protected, traceable, and secured against unintentional or unauthorized changes. An audit trail is therefore a proven means of meeting these requirements—but it is not an explicitly prescribed mandatory function.
Will ISO 17025 audits become stricter?
Yes, this development is clearly recognizable. A generational shift in auditors is leading to a more precise interpretation of ISO 17025. Younger auditors have grown up with digital standards and check data integrity, audit trails and calibration monitoring much more precisely than their predecessors. Laboratories should be prepared for the fact that manual or Excel-based solutions will be increasingly scrutinized.
How long does the changeover to digital test equipment management take?
The changeover typically takes two to six weeks. Data migration from Excel lists takes one to two weeks, software configuration three to five days. Added to this are one to two days of training and one to two weeks of parallel operation for validation. For laboratories with 100 to 500 pieces of test equipment, a realistic estimate is around four weeks. Larger laboratories or complex site structures may require more time.
Does every piece of test equipment have to have a unique number?
Yes, this is mandatory. § 6.4.3 of ISO 17025 requires a unique identification for each piece of test and measuring equipment. Duplicate or unclear numbers regularly lead to audit deviations. The identification must be permanently affixed to the device and prevent confusion. Even devices that have been removed from service must not pass on their numbers.
Which software is suitable for ISO 17025 test equipment management?
Suitable test equipment management software must offer several core functions: Unique IDs for all devices, automatic calibration reminders, complete audit trail, integrated document management for calibration certificates, status management with lock function and differentiated assignment of rights. Specialized solutions such as LabThunder or some LIMS systems with a test equipment module meet these requirements. Cloud or server-based systems also enable multi-site use.


LabThunder:
✅ Compliant with ISO 17025, GMP/GLP, and ISO 15189
✅ Digital logbooks instead of paper chaos
✅ Thunder AI central intelligence for errors & questions
✅ Smart & predictive maintenance prevents downtime
✅ Greater independence from external service providers
✅ Up to 50% fewer service calls
✅ Easy to use - no IT required
Contact us today for a free demo:
%20new.png)













.jpg)
.jpg)

