All articles
%20new.png)
From paper-based logbooks to digital GMP compliance: The perfect combination of test equipment management system and long-term archiving

Test equipment monitoring & laboratory digitization for standard-compliant implementation of ISO 17025 and ISO 15189.
Context does not arise from individual pieces of information, but from their controlled, semantic, and temporal linking.
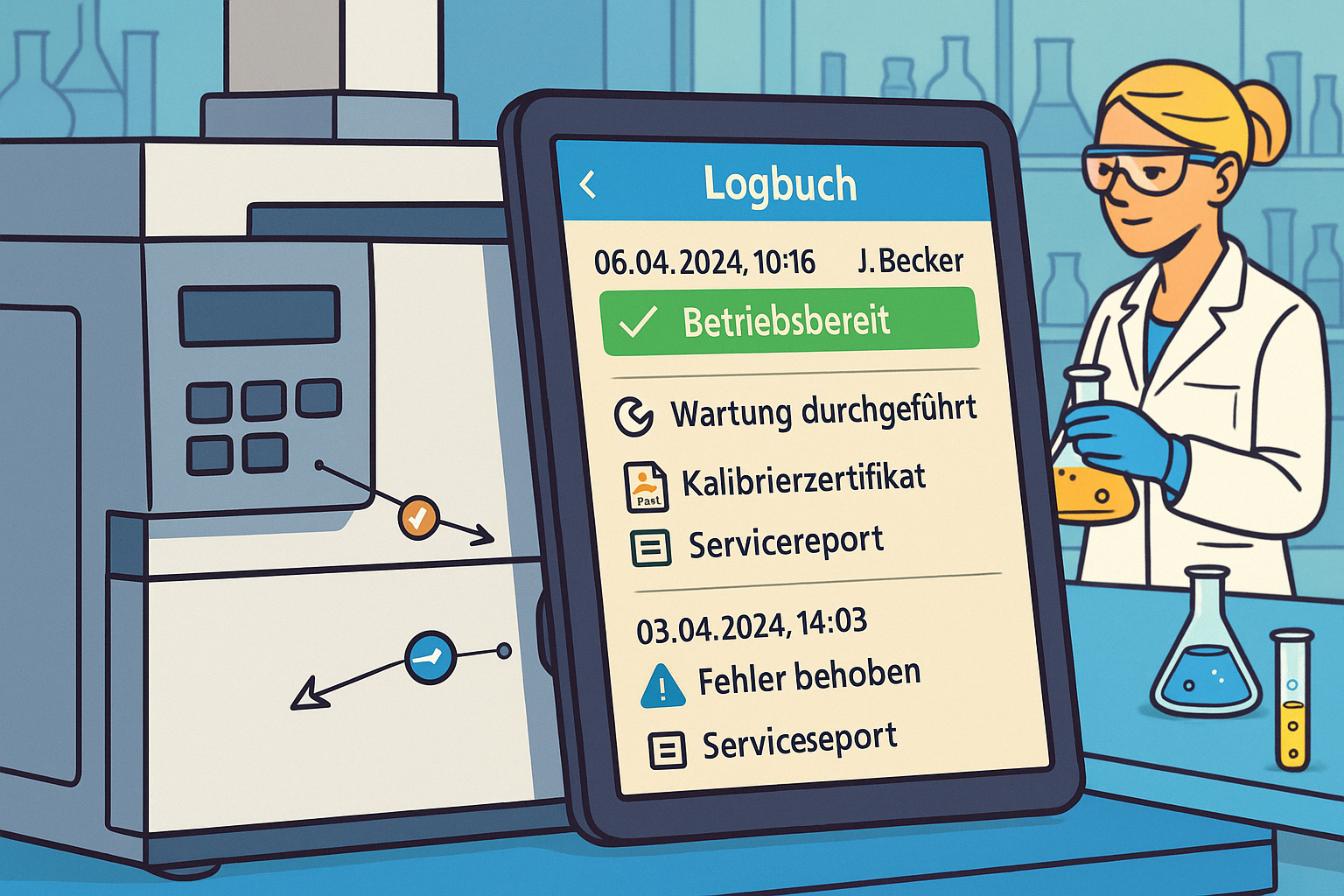
Digital logbooks in the laboratory: Why paper and Excel are no longer sufficient, what role ISO 17025, GMP, and ALCOA play—and how laboratories can use device knowledge in a future-proof way today.

Maintenance and calibration planning in the laboratory without chaos: How to comply with ISO 17025, GMP, and GLP and keep testing equipment, deadlines, and compliance under control.

A practical look at how AI reduces errors, makes knowledge available, and is changing everyday lab life for the long term.
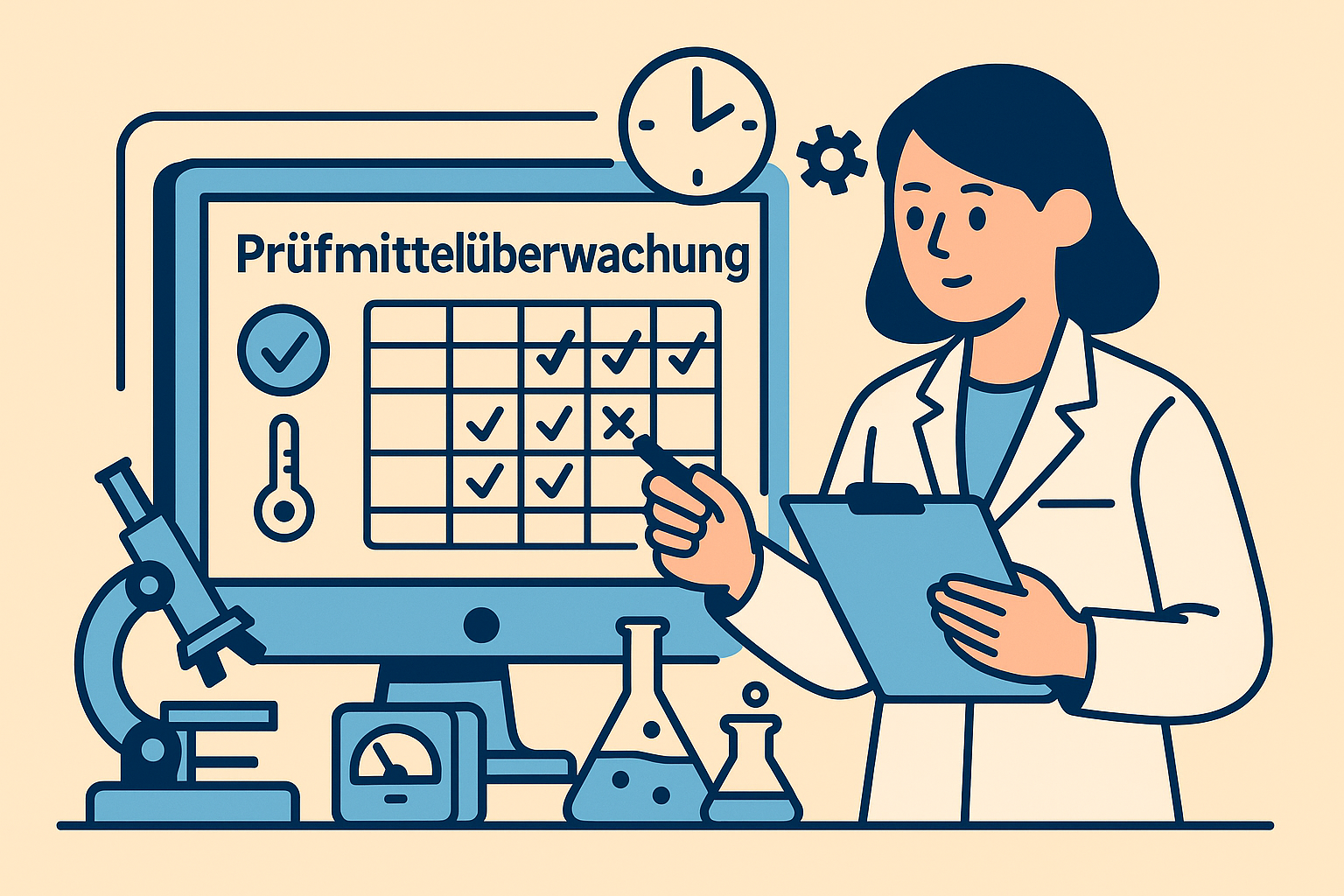
Test equipment monitoring in the laboratory: How to plan maintenance and calibration in accordance with ISO 17025, ISO 15189, GMP and GLP standards - without Excel chaos and deadline gaps.

Manage equipment errors professionally: Find out how digital test equipment management, laboratory digitization and AI-supported equipment management ensure ISO 17025, ISO 15189 and GxP compliance. ✓ Learn more

Shortage of specialists, complex analytics, dependent on individual experts? Find out how systematic knowledge retention can make your laboratory more stable, efficient and future-proof.