Introduction: Why two international standards pursue the same goal
Imagine this: An environmental laboratory measures the temperature of a water sample at exactly 23.4 °C. At the same time, a medical laboratory determines the body temperature of a patient - also at 23.4 °C (hypothetically, for the sake of comparison). Two completely different contexts, two different standards - and yet the same requirement: the result must be absolutely reliable.
Modern laboratories - whether testing, calibration or medical facilities - face the same challenges: reliable results, clear traceability, audit-compliant documentation and robust quality management. Although their tasks are fundamentally different, ISO 17025 (testing and calibration laboratories) and ISO 15189 (medical laboratories) are based on identical basic principles: Competence, validity, traceability, transparency and continuous improvement.
Both standards have the same overarching goal: to produce results that patients, customers and supervisory authorities can rely on. This article shows where the two standards meet, where they diverge - and how modern digitalization unites both worlds.

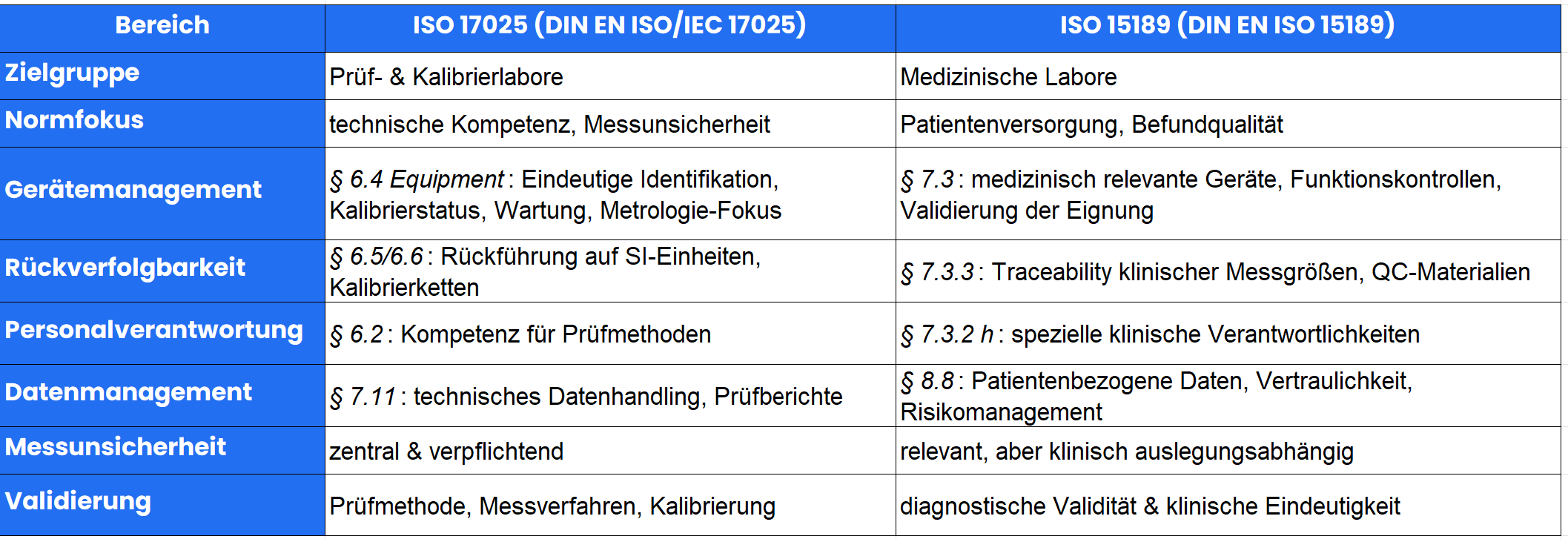
ISO 17025 vs. ISO 15189 - Areas of application and target groups
ISO 17025 / DIN EN ISO/IEC 17025
Applies to: Testing and calibration laboratories
Focus: technical competence, measurement uncertainty, test equipment management
Typical areas: Environmental analysis, material testing, physical and chemical measurements
The standard places a strong emphasis on:
- Traceability according to SI units (§ 6.5 / § 6.6)
- Requirements for test equipment and equipment (§ 6.4 Equipment)
- Validity and repeatability of results
Practical example: A calibration laboratory for industrial scales must be able to prove that every weight measurement is traceable to the international kilogram standard - with a documented calibration chain via national measuring institutes to the SI unit.
ISO 15189 / DIN EN ISO 15189
Applies to: medical laboratories
Focus: patient care, validation of findings, pre- & post-analytical processes
Incorporates ISO 9001 elements to a greater extent
In addition to technical expertise, special emphasis is placed on:
- clinical responsibility
- Interpretation of results
- Quality of patient care
Practical example: A clinical laboratory measures the HbA1c value of a diabetic patient. This is not only about the technical precision of the measurement, but also about the correct interpretation in the clinical context - was the sample taken correctly? Are the findings meaningful for the treatment decision? Was the patient prepared correctly?
Common quality principles
Despite different target groups, both standards are based on identical foundations. These similarities are no coincidence - they reflect universal quality requirements that apply to any form of precise measurement.
1. competence of the staff
ISO 17025 § 6.2: Qualification for testing and calibration tasks
ISO 15189 § 7.2 / § 5.1: Technical competence for medical findings
Both require documented competencies, training and regular effectiveness reviews.
In practice, this means that a laboratory technician who operates a mass spectrometer must provide proof of their qualifications, just like an MTA who prepares blood counts. Both have to undergo regular training, both undergo competence tests - and both leave their signatures in electronic systems that document exactly who carried out which measurement and when.
2. traceability - the core of both standards
Traceability means that every result can be clearly traced back to a valid reference - ideally SI units. This is the absolute foundation of both standards.
ISO 17025 § 6.5 / § 6.6: Traceability to national or international standards
ISO 15189 § 7.3.3: Traceability of clinical measurands
Why is this so critical? Imagine two laboratories measuring the same sample - and coming up with different results. Without traceability to common standards, it is impossible to tell which laboratory is correct. In the case of material testing, this could lead to production errors. In a medical diagnosis, a human life could depend on it.
Concrete example: A thermometer is calibrated at the national measuring institute. This institute in turn traces its measurements back to the international Kelvin standard. Every temperature measurement in your laboratory can now be traced back through this chain to the SI base unit - with documented measurement uncertainty at every stage.
3. documentation & data integrity
Both standards emphasize the need for auditable data. The focus is on four principles:
- Integrity: Data must not be changed unnoticed
- Completeness: No gaps in the documentation
- Protection against manipulation: changes must be traceable
- Audit trail: Who changed what and when - and why?
Reality check: An auditor appears in your laboratory and asks: "Show me the calibration history of the HPLC system from March 2023." Can you provide complete proof within 5 minutes? Both standards require exactly that - and modern digital systems make it possible.
4. quality management
Both standards prescribe a complete QM system:
- Document control
- Corrective actions (CAPA)
- continuous improvement
- Risk management
The difference to the "paper QM" of days gone by: in the past, this meant folders full of logs that nobody read. Today, it means intelligent workflows that automatically escalate when a device is overdue for calibration - before measurement errors occur.
Key differences at a glance
What both standards really have in common - the 6 quality pillars
Regardless of the laboratory order, both standards pursue the same quality objectives:
- Reproducible results - measurements must be repeatable
- Clear traceability of all measurements to recognized standards
- Safe device management - from commissioning to decommissioning
- Audit-compliant documentation - ready for audit at any time
- Risk and error reduction through preventive controls
- Clear responsibilities - no measurement without clear responsibility
This means that ISO 17025 and ISO 15189 are much closer to each other than they appear at first glance. They are two dialects of the same quality language.
Device management as a common key
Devices are the basis of every analytical measurement. An incorrectly calibrated pipetting system, an overdue thermometer, an analyzer without a maintenance history - all of these can falsify results. Both standards therefore define clear requirements:
1. clear labeling
Each device must be clearly identifiable:
- Asset ID (e.g. "HPLC-001")
- Calibration status (currently valid, overdue, blocked)
- Location (Laboratory A, Room 3.14)
- Responsible person (Dr. Müller)
ISO 17025 § 6.4.2 requires unique identification of all test equipment.
ISO 15189 § 7.3.2 requires a complete device file.
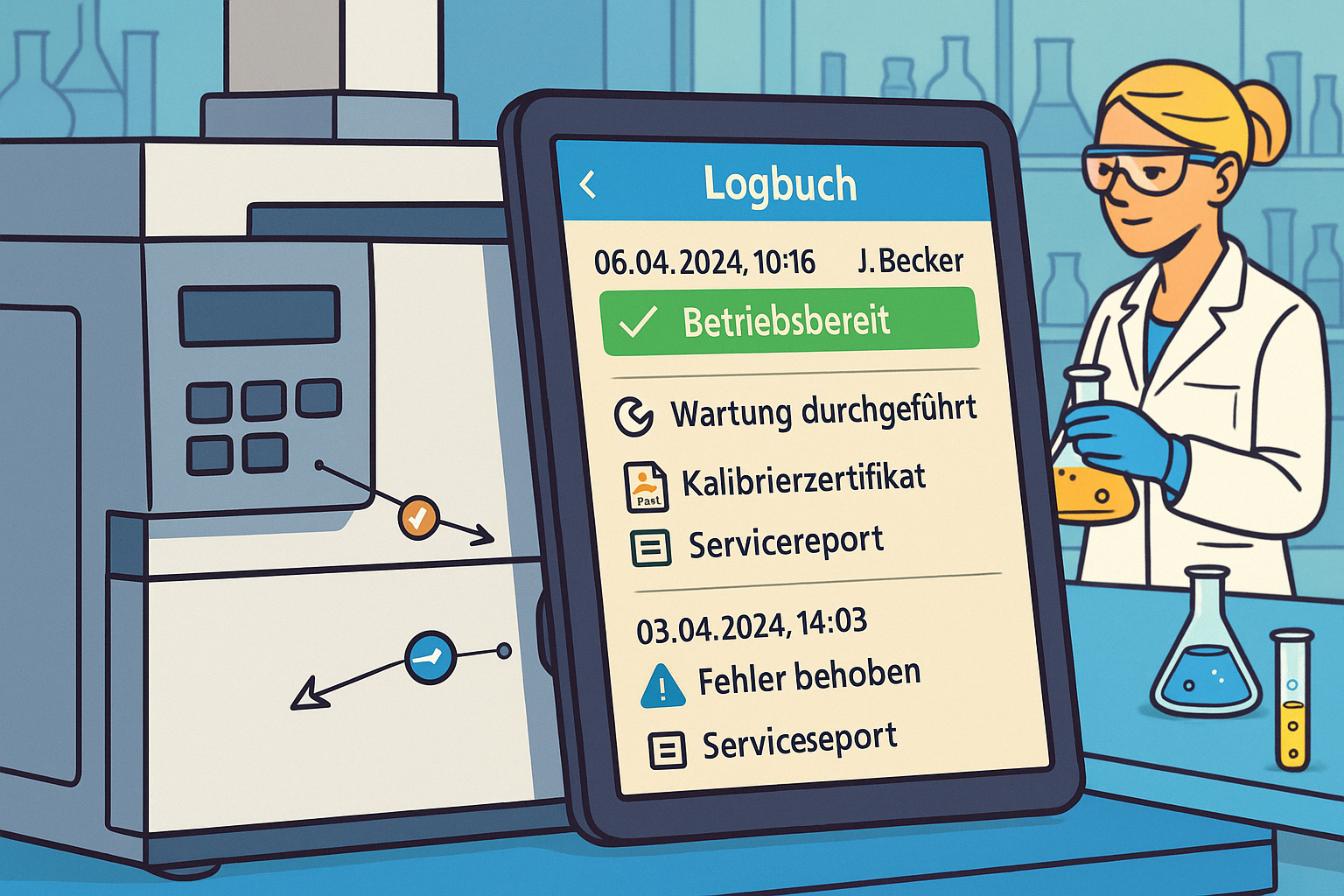
Practical scenario: An auditor enters your laboratory, points to an HPLC system and asks: "When was this device last calibrated? Who performed it? What deviations were there?" With digital identification (e.g. QR code), you scan the device and all the information appears on the tablet - in seconds.
2. calibration and traceability
Every measurement must be traceable to SI units - be it mass, time, temperature or volume.
The 7 SI base units:
- Meter (m) - Length
- Kilogram (kg) - mass
- Second (s) - Time
- Kelvin (K) - Temperature
- Mol (mol) - amount of substance
- Ampere (A) - electric current
- Candela (cd) - Luminous intensity
Practical example: A pharmaceutical company checks the volume of injection vials. The pipette used was tested with a calibrated reference volume. This reference volume was in turn certified by an accredited calibration laboratory, which traces its measurements back to national standards. The entire chain is documented - from the vial to the SI unit "liter".
3. maintenance and functional checks
Both standards require documented:
- Maintenance intervals (e.g. annually, semi-annually)
- Calibration cycles (based on manufacturer specifications or risk assessment)
- Functional tests (daily checks, e.g. temperature verification)
- Deviation management (What happens in the event of error messages?)
- Lock status in the event of malfunctions (defective devices may not be used)
Real case: An incubator suddenly shows 38 °C instead of 37 °C. Without the system, this might go unnoticed. With digital device management, the deviation triggers an alarm, the device is automatically locked and all measurements taken since the last successful check are marked for review.
4. device history & audit trail
So that auditors and QA managers can track every action:
- What has been changed? (Calibration certificate uploaded)
- When? (15.03.2025, 14:32)
- From whom? (Dr. Schmidt)
- On what grounds? (Annual calibration according to maintenance plan)
The difference between paper and digital: In a paper logbook, entries can be changed retrospectively - and nobody notices. A digital audit trail cannot be changed: every change is logged with a time stamp and user ID. This creates trust - with auditors, customers and authorities.
Digitization as a link - how modern laboratory software combines both standards
The requirements of ISO 17025 and ISO 15189 may seem complex on paper. In practice, many laboratories do not fail to understand the standards, but rather to implement them efficiently. This is where digitalization comes into play.
The challenge of the "paper laboratory"
Imagine a laboratory that still works with Excel spreadsheets, paper logbooks and manual reminders:
- A device is overdue for calibration - but no one has noticed because the Excel list has not been updated.
- An auditor asks about the maintenance history - and the team searches through three folders to find the relevant pages.
- A measurement must be traced - but the batch of reference material used was not noted.
This is not incompetence - this is everyday life in many laboratories that have not yet digitized. And this is exactly where modern laboratory software comes in.
Core topics that laboratory software must cover
1. digital test equipment management
- Central device files with all relevant information (manuals, certificates, service reports)
- Automatic calibration & maintenance reminders before deadlines expire
- Traceability of all activities through audit trail
- Use of SI units for calibration with documented measurement deviation
2. validated document control
- Electronic logbooks that automatically record time stamps and users
- Version control for SOPs and work instructions
- Automated reports for audits and management reviews
3. integration with laboratory processes
- Linking devices with specific measurements: Which device was used for which sample?
- Automatic plausibility checks: Was the device calibrated before the measurement?
- Real-time dashboards: Which devices are overdue? Where is there a need for action?
Why an integrated solution makes all the difference
Many laboratories work with several isolated systems: One software for device management, another for document control, Excel for calibration deadlines. The result: data silos, duplicate entries, sources of error.
An integrated solution, on the other hand, connects all processes:
Example workflow in a digitized laboratory:
- A new HPLC system is delivered
- It is registered in the software (asset ID, location, person responsible)
- The maintenance schedule is created automatically (calibration every 12 months, functional test daily)
- The system sends a reminder 30 days before the deadline expires
- The calibration is performed and the certificate is uploaded digitally
- The device is automatically enabled again - and all subsequent measurements are linked to it
- During an audit, the entire history can be exported with a single click
This is not a vision of the future - it is already a reality in digitized laboratories.
LabThunder: A platform for both worlds of standards
Digital solutions such as LabThunder show how the requirements of ISO 17025 and ISO 15189 can not only be met, but also implemented efficiently and error-free. The system was developed specifically for laboratory environments and connects:
- Digital test equipment management - central management of all devices, tools and measuring equipment
- Complete device histories - from commissioning to decommissioning
- Calibration tracking - automatic reminders, deadline management, certificate upload
- Digital logbooks - electronic records with audit trail
- Traceability of all activities - who did what and when?
- Role-based authorization management - not everyone can change everything
- Audit compliance for GxP, ISO and medical standards
LabThunder therefore covers all the key requirements of both standards - from unique identification and maintenance to fully auditable process chains. Whether environmental laboratory or clinic: the quality principles remain the same and the software adapts to the respective context.
Conclusion: Two standards, one goal - quality, competence and traceability
Even though ISO 17025 and ISO 15189 address different laboratory environments, they pursue the same goal: reliable results based on competent, traceable and audit-compliant processes.
Both standards are based on the same pillars:
- Measurement expertise - only qualified personnel carry out measurements
- Traceability - every result can be traced back to recognized standards
- Robust device management - devices are calibrated, maintained and monitored
- Quality-assured processes - documented, validated, audit-compliant
The real question is not whether your laboratory needs to meet these requirements - but how efficiently you meet them. Paper logbooks and Excel lists are not wrong - but they are error-prone, time-consuming and difficult to audit.
Through consistent digitalization - especially by means of integrated software solutions - these requirements can not only be met, but also implemented efficiently and in an audit-proof manner. Laboratories that take this step not only gain time and security - they also create the basis for excellent quality that patients, customers and supervisory authorities can rely on.
Because in the end, only one thing counts: the right result, at the right time, with complete traceability.

FAQ - ISO 17025 vs. ISO 15189
What is the main difference between ISO 17025 and ISO 15189?
ISO 17025 is aimed at testing and calibration laboratories and focuses on metrological competence, measurement uncertainty and technical validity.ISO 15189 is aimed at medical laboratories and also places strong emphasis on patient care, pre-/post-analysis and clinical evaluation.both standards are based on the same quality principles.
Do medical laboratories need ISO 17025 certification?
No. ISO 15189 is the relevant standard for medical laboratories.
ISO 17025 only becomes relevant if a medical laboratory carries out its own calibrations or metrological tests that must be verifiably traceable.
What does traceability mean in both standards?
Traceability means that every measurement can be traced back to a recognized reference standard - ideally to SI units (e.g. kg, K, s, mol).
- ISO 17025 § 6.5 / 6.6: complete calibration certificates
- ISO 15189 § 7.3.3: clinically relevant traceability, QC materials
Do both standards require documented device management?
Yes, both require clear identification, calibration and maintenance plans, functional checks and an audit-proof history.
- ISO 17025: § 6.4 Equipment
- ISO 15189: § 7.3
Digital device files make auditing much easier.
Does measurement uncertainty have to be calculated in both standards?
- ISO 17025: Yes, mandatory. Measurement uncertainty is a core requirement.
- ISO 15189: Yes, but context-dependent. It must be assessed for clinically relevant parameters, but is less formally structured.
What role does electronic documentation play?
Both standards require a system that:
- is comprehensible,
- tamper-proof,
- versioned,
- auditable,
- and audit-proof.
Electronic logbooks and validated laboratory software fulfill these requirements more reliably than paper files.
Are audit trails prescribed in both standards?
Indirectly yes.
While the standards do not necessarily use the word "audit trail", the requirements for data integrity, traceability and change tracking de facto presuppose such a system.
Why are SI units so important?
SI units (kilogram, meter, second, Kelvin, etc.) form the common language of all measurements.
Only SI-traceable calibrations provide true international comparability and auditable traceability - essential in both standards.


LabThunder:
✅ Compliant with ISO 17025, GMP/GLP, and ISO 15189
✅ Digital logbooks instead of paper chaos
✅ Thunder AI central intelligence for errors & questions
✅ Smart & predictive maintenance prevents downtime
✅ Greater independence from external service providers
✅ Up to 50% fewer service calls
✅ Easy to use - no IT required
Contact us today for a free demo:
%20new.png)













.jpg)
.jpg)

