In GMP- and GLP-regulated laboratories, test equipment management is a key compliance process. Every device used in the development, testing, or manufacture of pharmaceutical products must be fully traceable throughout its entire life cycle. At the heart of this documentation is the technical logbook—or, to use a more modern term, the TechLog.
The problem with paper-based test equipment management
Traditionally, technical logbooks are kept on paper. In many laboratories, there is a physical logbook next to each device in which all relevant activities are documented manually. What seems simple at first glance becomes a considerable burden over time:
Challenges in archiving
Paper logbooks are GxP-relevant documents and must be archived in the same way as batch records, analysis results, or validation documents. Specifically, this means:
- Retention periods of 10, 15, or even 20+ years
- Protection against loss, damage, or unauthorized changes
- Available at any time for audits and inspections
Laboratory equipment often remains in use for decades. During this time, a single paper logbook is often not enough—several logbooks are created for each device. With 100 devices in the laboratory, this quickly adds up to 100 to 200 physical logbooks that need to be archived.
The consequences:
- Growing archive volumes
- Rising storage and administrative costs
- Time-consuming audit preparation
- Increased compliance risk due to damaged or missing documents

Digitization in the laboratory is advancing steadily. Through the targeted use of digital lab books in conjunction with GxP-compliant archiving solutions, entire chains of paper-intensive processes can be eliminated.
The solution: Digital technical logbooks with LabThunder
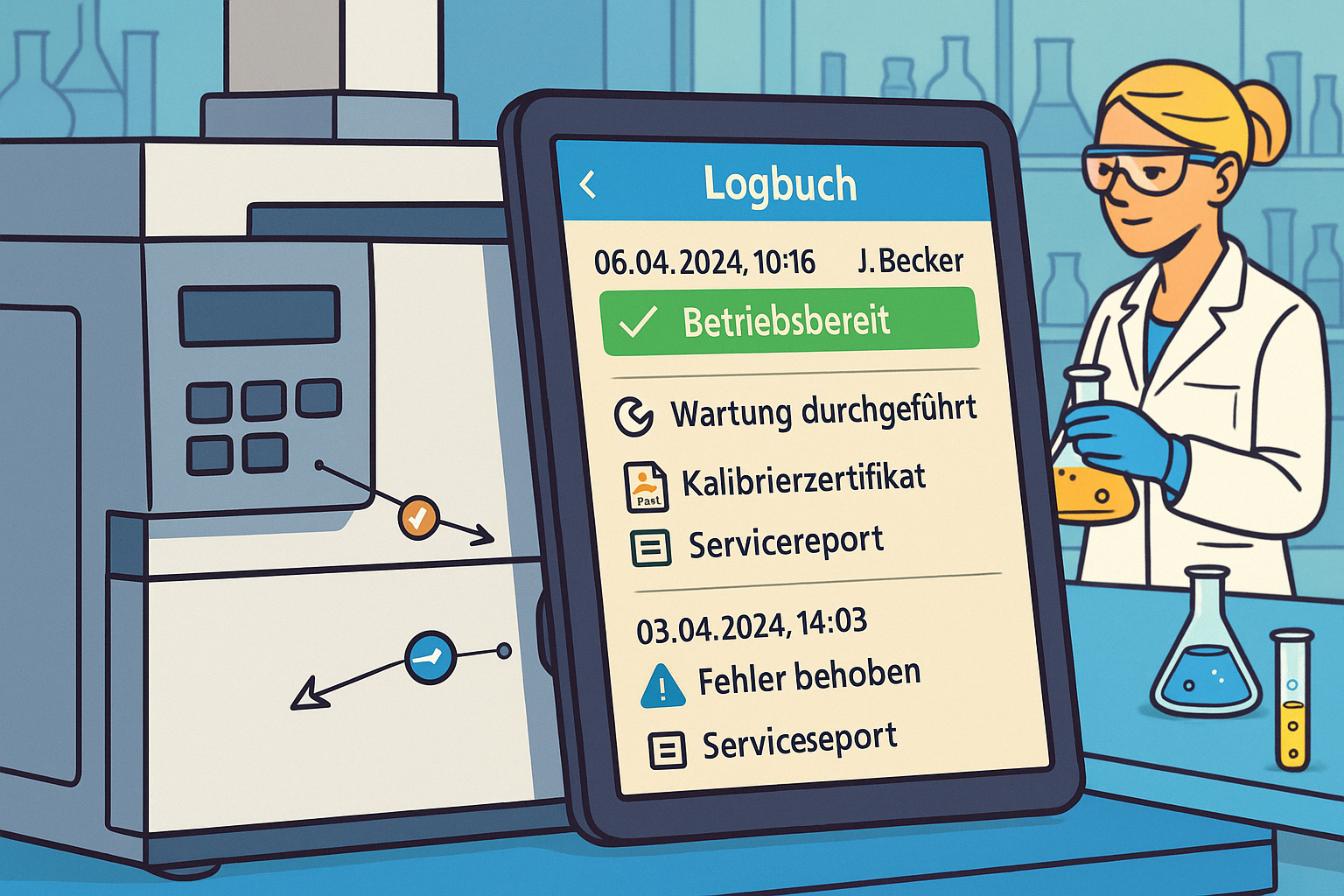
LabThunder is a fully digital system for technical logbooks, developed specifically for GMP and GLP-regulated environments. It replaces paper-based documentation with an electronic, structured, and compliance-compliant alternative.
What makes a digital logbook?
A digital technical logbook documents the entire life cycle of laboratory equipment:
- Installation and commissioning
- Qualification and validation
- Maintenance and calibration
- Repairs, deviations, and incidents
- Modifications, upgrades, and decommissioning
LabThunder this information in a format that:
- Searchable and structured is
- Consistently documented
- Access-controlled and traceable
- Ready for long-term digital archiving is
Test equipment management reimagined
The advantages of a digital technical logbook go far beyond mere documentation:
1. Efficiency in daily operations
- Quick access to all device information
- Automatic reminders for maintenance and calibration
- Central overview of the status of all test equipment
- No more illegible handwriting
2. Compliance and data integrity
- Complete audit trails for all changes
- Electronic signatures in accordance with 21 CFR Part 11
- Consistent documentation quality
- Reduced risk of compliance violations
3. Integration into modern laboratory IT
- Seamless connection to LIMS, QMS, and other systems
- Standardized processes across all locations
- Better data quality through structured entries
The perfect combination: LabThunder digital archiving
The real efficiency gains come from combining a digital logbook system such as LabThunder a GMP-compliant digital archiving solution.
When a device reaches the end of its life cycle, the complete digital logbook can be seamlessly transferred to a digital archive—for example, to biomedion's Watcher Archive.
This is how digital archiving works in practice:
- The device is being taken out of service.
- The digital TechLog is being finalized
- The logbook is transferred to the digital archive.
- Metadata is automatically enriched
- Retention periods and lifecycle rules are applied automatically
Measurable benefits for your laboratory
cost savings
- No more physical archive rooms for logbooks
- Reduced administrative costs through automation
- Less time spent on audits and inspections
compliance security
- Controlled lifecycle management
- Protection against data loss through redundant storage
- Audit-ready at all times thanks to instant access
scalability
- Unlimited scalability without additional physical space
- Future-proof regardless of system lifetimes
- Can be used across locations
From paper overload to digital efficiency
The transition from paper-based logbooks to a digital TechLog system such as LabThunder more than just a technological modernization—it is a strategic step toward more efficient test equipment management and more sustainable archiving processes.
The path to implementation
- Inventory: Recording of all currently used devices and logbooks
- System introduction: Step-by-step implementation of LabThunder
- Employee training: Training for consistent use
- Migration: Transfer of critical historical data
- Continuous optimization: Adapting to new requirements
Conclusion: Investing in the future
Digital technical logbooks are no longer a "nice-to-have"—they are a logical and necessary step toward efficient, compliant, and sustainable laboratory processes.
With LabThunder , regulated laboratories gain:
- Complete digital test equipment management
- Seamless archiving capability
- Long-term cost savings
- Increased compliance security
Instead of managing growing paper archives, companies get a scalable, compliant, and cost-effective strategy for their test equipment management.



LabThunder:
✅ Compliant with ISO 17025, GMP/GLP, and ISO 15189
✅ Digital logbooks instead of paper chaos
✅ Thunder AI central intelligence for errors & questions
✅ Smart & predictive maintenance prevents downtime
✅ Greater independence from external service providers
✅ Up to 50% fewer service calls
✅ Easy to use - no IT required
Contact us today for a free demo:














.jpg)
.jpg)

