Introduction – Why laboratory management software is indispensable today
Laboratories today face growing challenges: Quality assurance requirements are constantly increasing, qualified specialists are becoming scarcer, and the equipment landscape is becoming increasingly complex. At the same time, strict standards such as ISO 17025 and ISO 15189 must be complied with—often still using paper-based processes or disjointed Excel spreadsheets.
This is where modern laboratory management software comes in: it brings structure to daily workflows, creates transparency across all processes, and forms the foundation for sustainable quality assurance. Digitalization in the laboratory is no longer an optional extra, but a necessary basis for efficient, safe, and audit-proof work.
What is laboratory management software?
Laboratory management software is a central digital platform that brings together all essential aspects of laboratory organization: from resources and equipment to documents, responsibilities, and processes.
Important distinctions:
- Excel lists offer flexibility, but they are prone to errors, are not audit-proof, and do not allow for automation.
- LIMS (laboratory information management systems) focus primarily on the management of samples and analysis results.
- Laboratory management software extends this functionality to essential areas such as device management, maintenance planning, test equipment management, and the organization of roles and responsibilities.
It thus forms the organizational backbone of professional laboratory management and supports both managers and operational teams in their day-to-day business.
.png)
"Your lab, only smarter": With LabThunder , you always LabThunder all device information, maintenance schedules, and instructions at your fingertips—no searching, no chaos. LabThunder the next generation of laboratory management software. Our integrated Thunder AI not only Thunder AI operate complex systems, but also learns new things every day.
The role of laboratory digitization in the modern laboratory
Laboratory digitization means much more than simply doing away with paper. It is about intelligently linking information and making processes traceable.
Specific advantages in practice:
- Central data storage replaces fragmented isolated applications
- Quick and up-to-date access to device status and history
- Drastic reduction in manual transfer errors
- Clear transparency regarding responsibilities and accountabilities
- Time savings through automated routine tasks
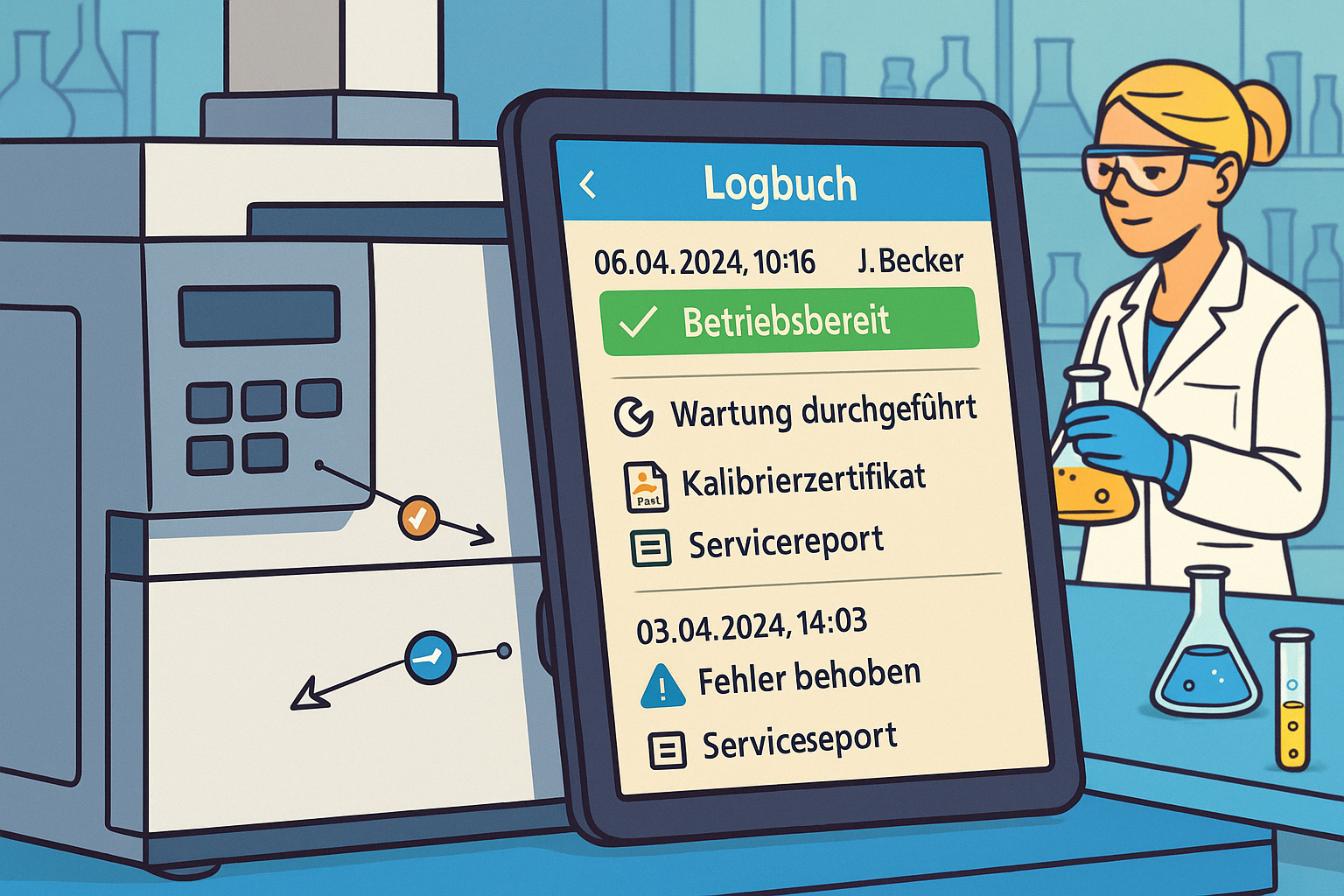
A key element here is lab equipment management: all information on devices, upcoming maintenance, calibrations performed, and any lockouts are traceable and fully documented at all times.
Test equipment management software & test equipment administration software
Test equipment management covers the entire life cycle of a measuring or test device in the laboratory:
- Procurement and initial commissioning
- Qualification for the intended area of application
- Regular calibration to ensure measurement accuracy
- Scheduled maintenance and repairs, if necessary
- Documented decommissioning
Test equipment management software digitally maps this entire cycle. It not only records the current status, but also documents the history, responsibilities, and all relevant status changes.
Digital test equipment files typically contain:
- Calibration certificates with all technical details
- Proof of maintenance performed
- Defined areas of application and measuring ranges
- Documented deviations and measures taken
- Release status and usage restrictions
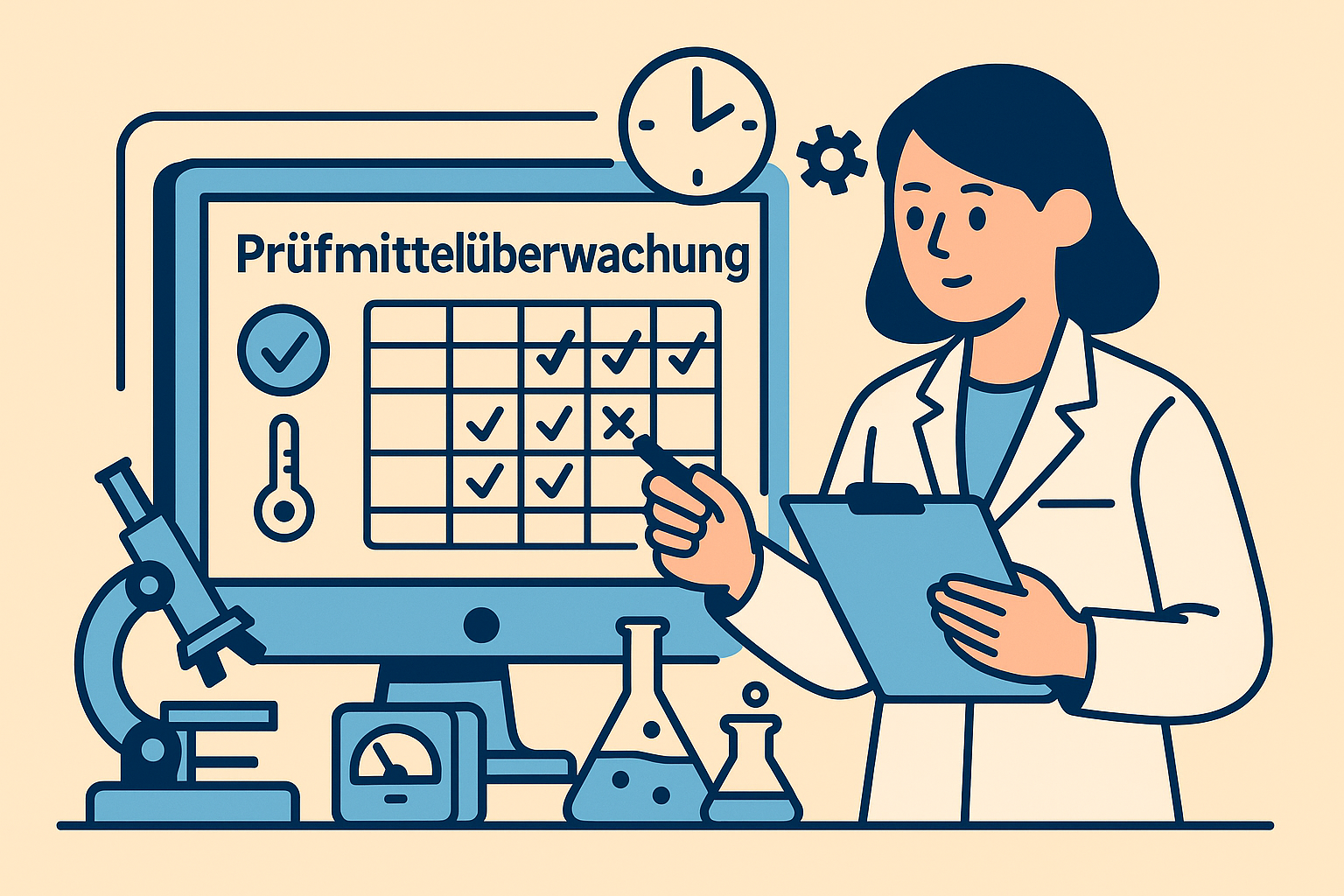
Test equipment monitoring & calibration – compliant with standards & audit-proof
Systematic test equipment monitoring is a key requirement of the ISO 17025 and ISO 15189 standards. Specialized test equipment monitoring software supports laboratories on several levels:
- Automatic deadline monitoring: The system continuously monitors all calibration deadlines and notifies you of upcoming dates in good time.
- Early warnings: Laboratory staff receive notifications before deadlines actually expire.
- Automatic lockouts: If calibration has expired, the test equipment is automatically locked out for use.
- Complete documentation: Every action and every status change is automatically logged.
This ensures that only approved testing equipment that is suitable for the respective purpose is used—a decisive factor for valid measurement results and security during audits.
Risks without digital laboratory management software
Laboratories that operate without structured laboratory management software expose themselves to several risks:
Excel chaos: Different versions and conflicting data lead to uncertainty and wrong decisions.
Lack of traceability: Calibration histories and maintenance records cannot be quickly and completely verified.
Audit Findings: During inspections and audits, incomplete or inconsistent documentation stands out negatively.
Unnecessary costs: Unplanned downtime, missed maintenance appointments, and emergency calibrations result in avoidable expenses.
Quality and safety risks: The use of uncalibrated or unsuitable test equipment jeopardizes the reliability of measurement results.
Advantages of modern laboratory management software
A modern solution for laboratory management offers measurable added value at various levels:
Efficiency gains: Significantly reduced search times, clear processes, and automated routine tasks noticeably reduce the workload for the team.
Process clarity: Defined responsibilities and documented procedures create security for all involved.
Audit security: Structured, readily available evidence makes internal and external audits much easier.
Compliance support: The software actively assists in meeting the requirements of ISO 17025 and ISO 15189.
Automated test equipment monitoring: Calibration intervals and maintenance dates are reliably monitored without the need for manual checks.
Better collaboration: Transparent information promotes communication and coordination within the team.
Important features laboratories should look out for
When selecting suitable laboratory management software, the following core functions should be taken into account:
Centralized, structured database: All relevant information is available in one place and is consistent.
Calibration and maintenance planning: Automatic planning and reminders for upcoming appointments.
Role and rights management: Differentiated access rights according to responsibilities.
Audit trail and change tracking: Every change is logged with a timestamp and user name.
Integrated document management: Certificates, reports, and documentation are directly linked to the devices.
Interfaces to existing systems: Connection to LIMS, ERP, or other specialist systems for end-to-end processes.
ISO 17025 & ISO 15189 – how software supports implementation
Both standards set clear requirements for laboratory organization:
- Use of suitable and regularly monitored testing equipment
- Documented and traceable processes
- Transparent decision-making processes
- Clearly defined roles and responsibilities
Laboratory management software provides significant support in the practical implementation of these requirements: it automatically provides the necessary evidence, standardizes processes across the entire laboratory, and immediately highlights any deviations—without creating additional documentation work. This not only makes day-to-day work easier, but also provides the assurance that all relevant information is readily available during audits.
Introduction & Change Management
The success of new software depends not only on its functions, but above all on the people who work with it:
Early involvement: Involve your employees as early as the planning phase and listen to their practical experiences.
Clear communication: Explain in understandable terms the specific benefits the software brings to everyday laboratory work.
Targeted training: Provide sufficient time and resources for familiarization and training.
Joint process definition: Develop the new digital processes together with the team, not over their heads.
When digitalization is perceived as a genuine relief rather than a control mechanism, acceptance increases significantly.
Practical examples / Use cases
Example 1: Calibration period expires
The system automatically recognizes that the calibration of an important measuring device will expire in three weeks. The responsible employee receives a notification and can plan the appointment in good time—instead of having to react at the last minute.
Example 2: Device is locked
A test device has exceeded its calibration period. The software automatically locks it for further measurements. This prevents incorrect results from being produced. It is only released again after successful recalibration.
Example 3: Audit preparation
An external audit is imminent. Thanks to digital laboratory management, all test equipment files, calibration certificates, and maintenance records are available with just a few clicks. The auditors receive complete and consistent documentation.
Conclusion
Laboratory management software is now an indispensable component for laboratories that want to work efficiently, safely, and in compliance with standards. It combines laboratory digitization, lab equipment management, and test equipment monitoring into a consistent overall system.
If you want to ensure long-term quality, reduce costs, and pass audits with confidence, structured digital laboratory management is essential. Investing in professional test equipment management software and a well-designed test equipment management system quickly pays off through greater transparency, fewer errors, and increased efficiency.

What is laboratory management software?
Laboratory management software is a digital platform for the centralized organization of all laboratory processes, resources, equipment, documents, and responsibilities. It goes beyond simple sample management and also includes equipment management, maintenance planning, and quality assurance.
What does test equipment management involve?
Test equipment management covers the entire life cycle of a measuring or test device: from procurement to qualification, regular calibration and maintenance, and decommissioning. All steps are documented and made traceable.
What role does ISO 17025 play in the laboratory?
ISO 17025 is the international standard for the competence of testing and calibration laboratories. It defines requirements for quality management, technical competence, and the reliability of test results. Accreditation according to ISO 17025 is required in many areas.
What does test equipment monitoring mean?
Test equipment monitoring refers to the systematic control of all measuring and testing equipment with regard to their calibration intervals, technical condition, and suitability for the intended use. The aim is to use only validated and approved devices.
How does software help with ISO 15189 compliance?
ISO 15189 applies specifically to medical laboratories. Laboratory management software supports compliance by standardizing processes, automatically documenting evidence, monitoring devices, and clearly defining responsibilities. This greatly facilitates both daily work and preparation for audits.


LabThunder:
✅ Compliant with ISO 17025, GMP/GLP, and ISO 15189
✅ Digital logbooks instead of paper chaos
✅ Thunder AI central intelligence for errors & questions
✅ Smart & predictive maintenance prevents downtime
✅ Greater independence from external service providers
✅ Up to 50% fewer service calls
✅ Easy to use - no IT required
Contact us today for a free demo:
%20new.png)













.jpg)
.jpg)

