Maintenance and calibration planning in the laboratory: why Excel lists are no longer enough
In many laboratories, maintenance and calibration appointments are still organized using Excel lists, Outlook reminders or handwritten notes. As long as only a few devices are in use, this usually works quite well. However, as soon as the laboratory grows, accreditation in accordance with DIN EN ISO/IEC 17025 is pending or you are working in a GMP laboratory or GLP laboratory, the weaknesses of these isolated solutions become clear: deadlines are overlooked, responsibilities remain unclear, evidence is incomplete or scattered, and the auditor does not find consistent documentation during the audit.
This results in gaps in the monitoring of measuring equipment, non-traceable calibrations, unplanned downtimes and critical discussions with auditors or authorities. Professional maintenance and calibration planning is therefore not a "nice-to-have", but essential for functioning laboratory management. Especially in the context of rising regulatory requirements and increasing digitalization, structured, audit-proof equipment management is needed in order to be able to prove the functionality and traceability of all relevant testing and measuring equipment at all times.

Maintenance and calibration planning doesn't have to be complicated. LabThunder gives you a structured and transparent overview of your entire equipment fleet at all times: Color codes show the respective planning status at a glance, and with just one click on the tile you can jump directly to the appropriate planning view of the system.
What standards and regulations actually require
Anyone working in the accredited or regulated sector operates within a clearly defined framework. DIN EN ISO/IEC 17025 explicitly requires all measuring and testing equipment to be suitable, regularly monitored and maintained. Calibrations must be traceable to national or international standards and the status of each device must be recognizable at all times. Documentation, status labeling and evaluation of measurement uncertainty are key aspects of test equipment monitoring calibration.
DIN EN ISO 15189 sets similar requirements for medical laboratories, where patient-related measurements are also involved. Traceability, validity of results and structured monitoring of measuring equipment are particularly critical here.
In GMP laboratories and GLP laboratories, things are even stricter: strict rules apply to device qualification, status labeling, documentation and approval. Devices may only be operated in an approved condition and all measures must be documented in a traceable and verifiable manner.
Data integrity according to the ALCOA principle is a cross-cutting issue. It requires that all entries relating to maintenance, servicing, qualification and calibration must be assignable, legible, promptly recorded, available in the original and correct - supplemented by completeness, consistency, durability and availability. This means that poorly maintained Excel lists and loose pieces of paper in folders are generally no longer sufficient.
Risk-based thinking: not all devices are equally important
Modern measuring equipment monitoring follows a risk-based approach. First, the criticality of the devices is assessed: Which ones have a direct impact on clearance decisions, patient outcomes or regulatory relevant testing? These devices require tighter intervals and stricter traceability and documentation requirements.
The definition of maintenance and calibration intervals is based on manufacturer specifications, empirical values, failure history and process relevance. It is important to review these intervals regularly based on deviations and trend analyses.
Traceability and validity are key: calibrations of critical test equipment must be traceable to national or international standards - this is a core requirement of DIN EN ISO/IEC 17025. The status of the test equipment (released, blocked, out of service) must be recognizable at all times.
After each calibration, a documented assessment is made as to whether the test equipment is still suitable for its purpose. Decisions and assessments must be documented in a comprehensible manner as part of the test equipment management system.
How to proceed systematically
The creation of a structured system follows a clear plan. First, all relevant testing and measuring equipment is inventoried with clear identification (ID, location, person responsible) and allocation to processes, methods and products.
Criticality is then classified into classes (high, medium, low) according to the impact on quality of outcome and patient safety. Measures and intervals are defined for each class: Scope of maintenance, scope of calibration, qualification requirements and documentation requirements.
Documentation should be standardized - using uniform forms or digital forms for maintenance reports, calibration protocols and qualification documents. Although the ALCOA principle is explicitly required above all in GMP and GLP laboratories, the requirements for data integrity are also constantly increasing in ISO-accredited laboratories. The advantage of modern software is that it often implements these principles - assignable entries, time stamps, electronic signatures - in passing, without making things complicated for users. Data integrity does not have to mean bureaucracy. Those who rely on a well thought-out system today are also well equipped for future requirements - regardless of whether they work in accordance with DIN EN ISO/IEC 17025, DIN EN ISO 15189 or in the regulated area.
The decisive step is the introduction of a central system: replacing distributed Excel files with a structured solution for lab equipment management. As part of digitalization, the use of a central platform that links maintenance, calibration and qualification data with SOPs, logbooks and quality documents makes sense.
Finally, training and role clarification with defined responsibilities for test equipment management, maintenance coordination and approval as well as regular training for laboratory managers, QA/QM and technical staff are required.
Planning with an eye on the big picture: deadlines and resources
Good planning not only takes into account deadlines, but also resources. Annual planning brings together all maintenance, calibration and qualification dates in a central calendar, taking into account peak load phases, vacation periods and ongoing projects.
Resource balancing ensures that sufficient alternative devices or capacities are available during maintenance. Redundancies or emergency plans should be defined for critical devices.
Status management ensures clear labeling of devices (e.g. "in maintenance", "calibrated until...", "locked"). Integration into laboratory management software means that all employees can see the status at any time. This minimizes downtimes and avoids bottlenecks in routine operations.
Digitalization as a sustainable path
In the course of digitalization, more and more laboratories are relying on specialized test equipment management software and test equipment management software. These systems support the central management of all test and measuring equipment, automatic reminders of maintenance and calibration deadlines, the storage of certificates, service reports and qualification documents as well as status identification and audit trail functionalities.
For smaller laboratories, simple or even free test equipment monitoring software solutions can be a good start. They at least replace the fragmented Excel test equipment monitoring system and create an initial structure for equipment management.
For regulated environments with GMP laboratories or GLP laboratories, a professional system is usually required that offers ALCOA-compliant logging, user and rights concepts as well as interfaces to other systems. Modern laboratory management software combines test equipment monitoring, measuring equipment management and equipment history with SOPs, logbooks and quality documents, thus supporting integrated equipment management.
What has proven itself in practice
Some tried and tested principles have become established. "Single source of truth" means that all information on test equipment monitoring, maintenance, qualification and calibration is maintained in one place.
Roles and responsibilities should be clearly defined: laboratory manager, QA/QM and technology should have clearly assigned tasks and powers. Standardized workflows ensure uniform processes for maintenance, calibration, qualification, verification and release decisions.
The link with quality management ensures that deviations from calibrations (such as the unsuitability of a device) are automatically incorporated into CAPA or deviation processes. Regular reviews - an annual review of intervals, service providers, costs and downtimes - enable continuous improvement.
The result is a consistent system that meets the standard requirements of DIN EN ISO/IEC 17025, DIN EN ISO 15189 and the specifications for GMP laboratories and GLP equipment.
Why the effort is worth it
Efficient maintenance and calibration planning is a central element of professional laboratory management. It protects against failures, reduces the risk of incorrect measurement results and provides security for auditors and authorities.
Those who consistently move away from isolated solutions such as Excel test equipment monitoring and rely on structured, digital lab equipment management not only meet the requirements of ISO 17025, ISO 15189, GMP and GLP, but also increase efficiency and transparency in everyday laboratory work.
Well thought-out test equipment management with clearly defined processes, risk-based planning, digital test equipment monitoring and traceable documentation is therefore not an additional expense, but the basis for a robust, future-proof and audit-proof laboratory. Whether an internally developed solution or a specialized platform is used for this is of secondary importance - the decisive factor is that maintenance, calibration and test equipment management are organized in a consistent, traceable and audit-proof manner.

Frequently asked questions about maintenance and calibration planning / test equipment monitoring
What is test equipment monitoring in the laboratory?
Test equipment monitoring includes all measures that a laboratory uses to ensure that measuring and test equipment is functional, suitable and compliant with standards at all times. This includes inventory, labeling, maintenance, calibration, documentation and the clear definition of the device status (released, blocked, out of service). In accredited laboratories in accordance with DIN EN ISO/IEC 17025 or DIN EN ISO 15189, systematic test equipment monitoring is an integral part of quality management.
How often does test equipment need to be maintained and calibrated?
There are no standardized intervals. The frequency of maintenance and calibration depends on the criticality of the device, the frequency of use, the manufacturer's specifications, previous faults and the normative requirements (e.g. ISO 17025, ISO 15189, GMP, GLP). It is important that the laboratory justifies and documents the intervals and regularly checks whether they are still appropriate.
Why is test equipment monitoring with Excel often problematic?
Excel lists seem flexible at first, but quickly become confusing as the number of devices increases. Typical problems include version conflicts, typing errors, a lack of overview of deadlines and no traceable audit trail. From a data integrity perspective (e.g. ALCOA principle), it is difficult to prove who made which changes and when. At the latest during the audit, it becomes clear that a pure Excel solution is only of limited use.
What advantages does specialized software offer over Excel?
A special solution for test equipment and device management can bundle master data, maintenance and calibration dates, protocols, certificates and device status in one place. Reminders run automatically, the current status is visible to everyone and changes are logged in a traceable manner. This makes it easier to comply with standards such as ISO 17025, ISO 15189, GMP and GLP and reduces the organizational effort in everyday life.
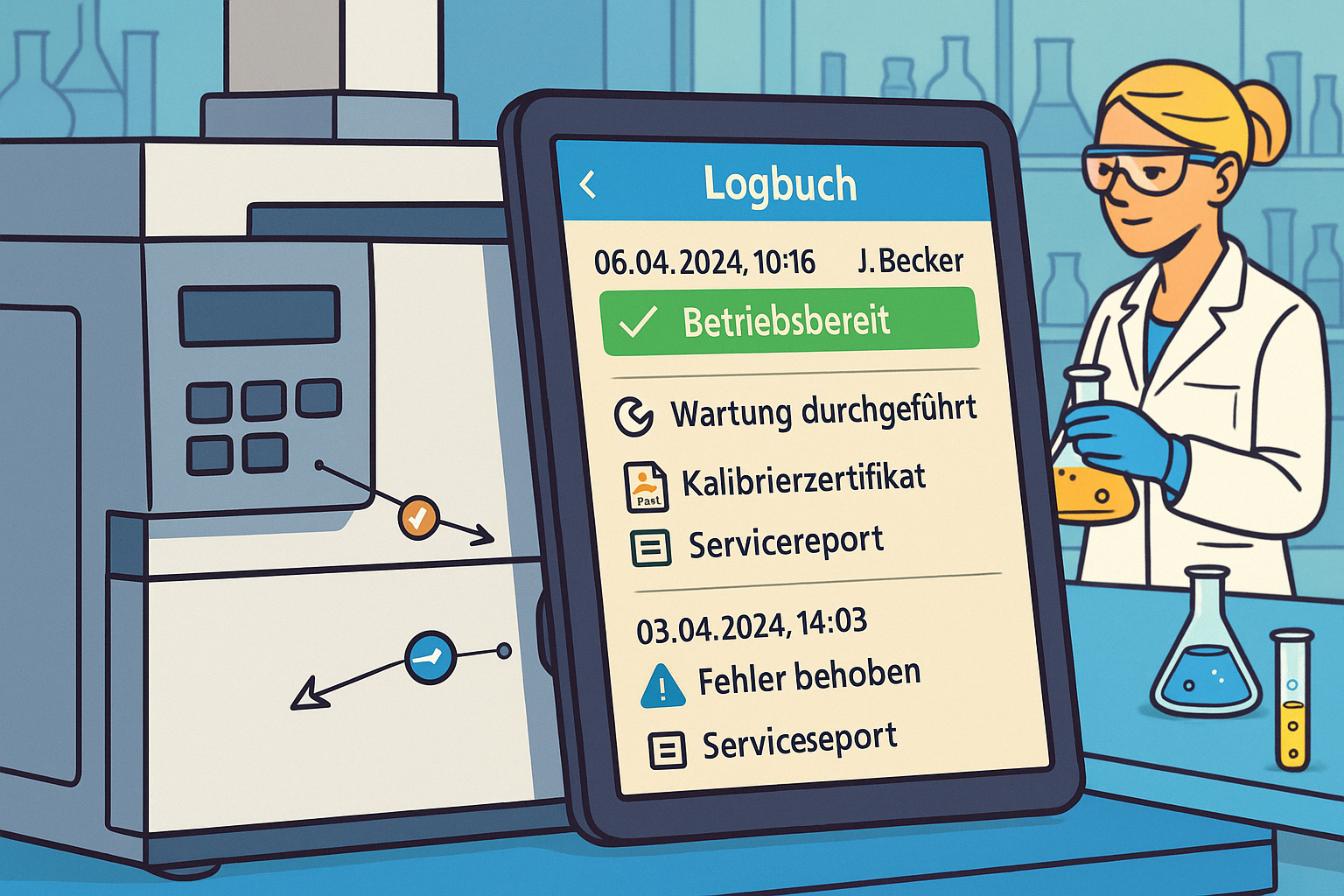
How does LabThunder support maintenance and calibration planning?
LabThunder combines device management, digital logbooks and test equipment monitoring in one interface. The entire equipment fleet is displayed in the form of tiles, different colors show the respective planning status, and one click takes you directly to the planning view of the system concerned. This allows laboratory management, quality assurance and technology to maintain an overview at all times and control maintenance, calibration and faults in a structured and audit-proof manner.


LabThunder:
✅ Compliant with ISO 17025, GMP/GLP, and ISO 15189
✅ Digital logbooks instead of paper chaos
✅ Thunder AI central intelligence for errors & questions
✅ Smart & predictive maintenance prevents downtime
✅ Greater independence from external service providers
✅ Up to 50% fewer service calls
✅ Easy to use - no IT required
Contact us today for a free demo:
%20new.png)














.jpg)
.jpg)

