Introduction
Equipment errors are among the most common causes of deviations, quality problems and unplanned downtime in analytical and diagnostic laboratories. Whether in GMP laboratories, GLP laboratories or in facilities accredited according to DIN EN ISO/IEC 17025 or ISO 15189 - the structured handling of equipment errors is crucial for valid results and consistent data integrity.
Nevertheless, many laboratories continue to rely on Excel lists, decentralized documentation or paper logbooks for test equipment management, measuring equipment monitoring and measuring equipment management. This approach entails considerable risks: lack of versioning, no audit trails, inconsistent nomenclature, media disruptions and difficult trend analyses.
As regulatory complexity increases, laboratory digitalization, professional lab equipment management, laboratory management software and process-oriented test equipment management software are becoming increasingly important - not only to increase efficiency, but above all to ensure compliance.

What are equipment faults?
Equipment faults occur on measuring devices, test equipment, laboratory instruments or automated systems and can be divided into several categories.
Technical error messages
These include pump failures, valve faults and pressure instabilities as well as temperature drift in incubators or ovens. Sensor faults such as UV lamp ageing or detector drift also fall into this category. These technical faults often require an immediate response and can completely interrupt measuring operations.
Deviating measured values & drift
Unexpected standard deviations, implausible peaks or signals and unstable baselines indicate systematic problems. These errors directly affect test equipment monitoring and calibration and may require re-qualification.
Calibration error
Overdue calibration deadlines, missing verifications or incorrectly applied calibration procedures represent a significant compliance risk. The requirements for test equipment monitoring calibration are described in detail in DIN EN ISO IEC 17025 (also known as DIN ISO EN 17025, DIN EN ISO 17025 or DIN ISO IEC 17025) and must be implemented consistently.
Documentation errors (ALCOA principle)
Typical problems with paper-based or Excel-based documentation (inspection equipment monitoring Excel) are missing time stamps, unclear assignments, subsequent changes without an audit trail and missing assignment of persons. These deficiencies contradict the ALCOA principle and jeopardize data integrity.
Operating error
Unclear SOPs, incorrect parameterization or a lack of training often lead to recurring errors. This category clearly shows the importance of systematic laboratory management, structured knowledge management and, in particular, professional laboratory knowledge management to avoid operating errors.
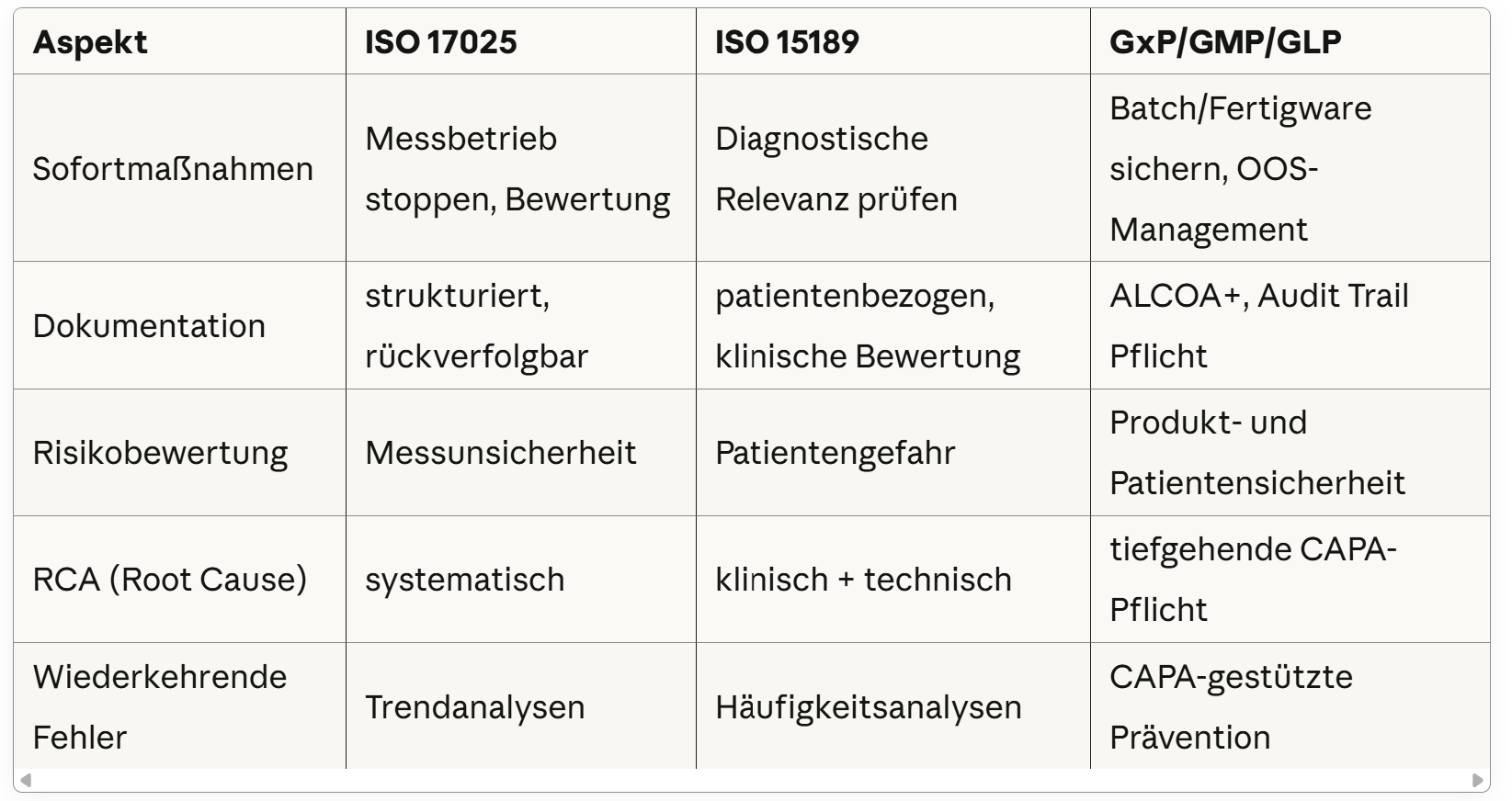
Tabular comparison of the differences in error management between ISO 17025, ISO 15189 and GxP/GMP/GLP - each with a focus on immediate measures, documentation, risk assessment, root cause analysis and recurring errors.
The three standard systems at a glance
DIN EN ISO/IEC 17025
The focus is on measuring equipment monitoring, test equipment management including traceability, regular calibration and verification as well as the documentation of deviations. The evaluation of measurement uncertainty is also required. The handling of equipment errors must be documented in a comprehensible, assessable and reproducible manner. The standard is often also referred to as DIN ISO 17025 or DIN EN ISO 17025.
DIN EN ISO 15189
Special requirements apply to medical laboratories due to the direct impact on patient safety and the validity of diagnostic results. There are stricter requirements for response times, assessment and CAPA as well as a close link between equipment errors and clinical decisions.
GxP/GMP/GLP
The GxP regulations expect complete data integrity in accordance with ALCOA+, seamless audit trails, qualified suppliers and documented equipment qualifications (IQ/OQ/PQ). Both GMP laboratories and GLP laboratories require structured test equipment management and documentation of GLP equipment with complete life cycles. Errors must be documented in digitally tamper-proof systems in a traceable manner.
Common basic logic of all standards
Despite their different focuses, all three systems follow a uniform core process:
- Recognize errors
- Stop or isolate the device
- Assessment (risk, validity, impact)
- Root cause analysis (RCA)
- Corrective measures
- Preventive measures (CAPA)
- Effectiveness test
- Documentation in the audit trail
These steps form the basis for digital equipment management, structured test equipment management, professional test equipment management software and modern laboratory management.
Modern solutions
Laboratory digitization
Digital systems automate documentation, link measured value data with logbooks and device status and support standard-compliant processes. They eliminate media discontinuities and create end-to-end transparency.
Digital test equipment management software
Professional test equipment management software automatically monitors calibration deadlines, keeps a complete history, proactively points out risks and replaces error-prone Excel solutions. While free test equipment monitoring software can close initial gaps free of charge, it quickly reaches its limits with GxP audits in terms of audit trail, validation and data integrity.
Automated measuring equipment monitoring
Modern systems integrate real-time data, maintenance cycles, calibration intervals, usage data and drift analyses in a central platform. This enables a proactive rather than reactive maintenance strategy.
Predictive maintenance
By analyzing usage data, errors and deviations can be predicted before they actually occur. This significantly reduces unplanned downtimes and improves predictability.
Lab Equipment Management Systems
These systems form the center of modern laboratory management with central asset management, tamper-proof audit trails, real-time status monitoring and digital logbooks.
How LabThunder manages equipment faults
LabThunder combines laboratory digitization, equipment management, test equipment management software and AI-supported troubleshooting in a standard-compliant approach for ISO 17025, ISO 15189 and GxP laboratories.
Early detection of device faults
The system continuously records device status, usage data and logbook entries. Automatic detection of conspicuous trends (e.g. detector drift, pressure increase or unusual peaks) allows potential problems to be identified at an early stage.
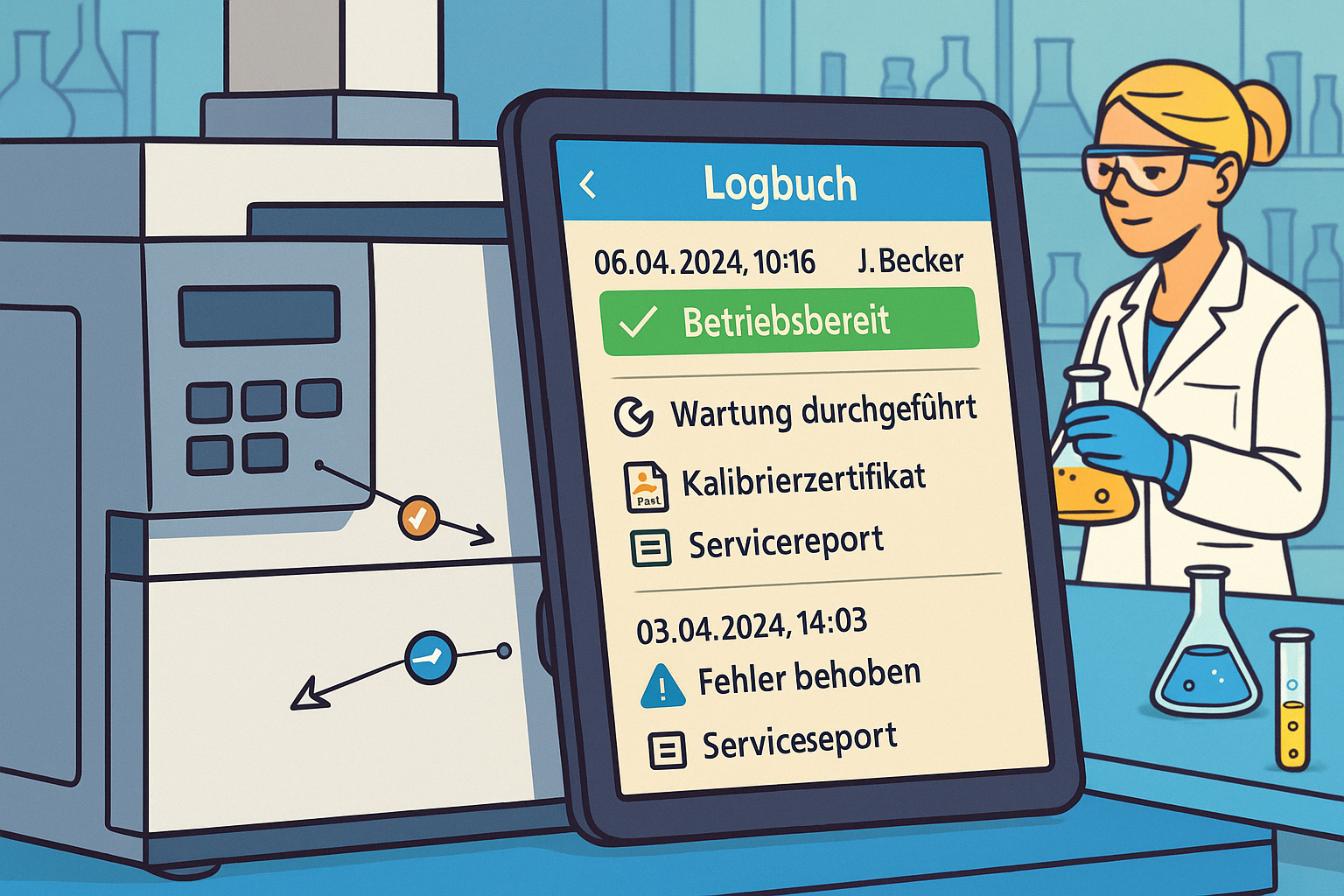
Digital logbooks
LabThunder offers tamper-proof audit trails according to the ALCOA principle, structured recording of faults, deviations and measures as well as a direct link to calibration and maintenance history.
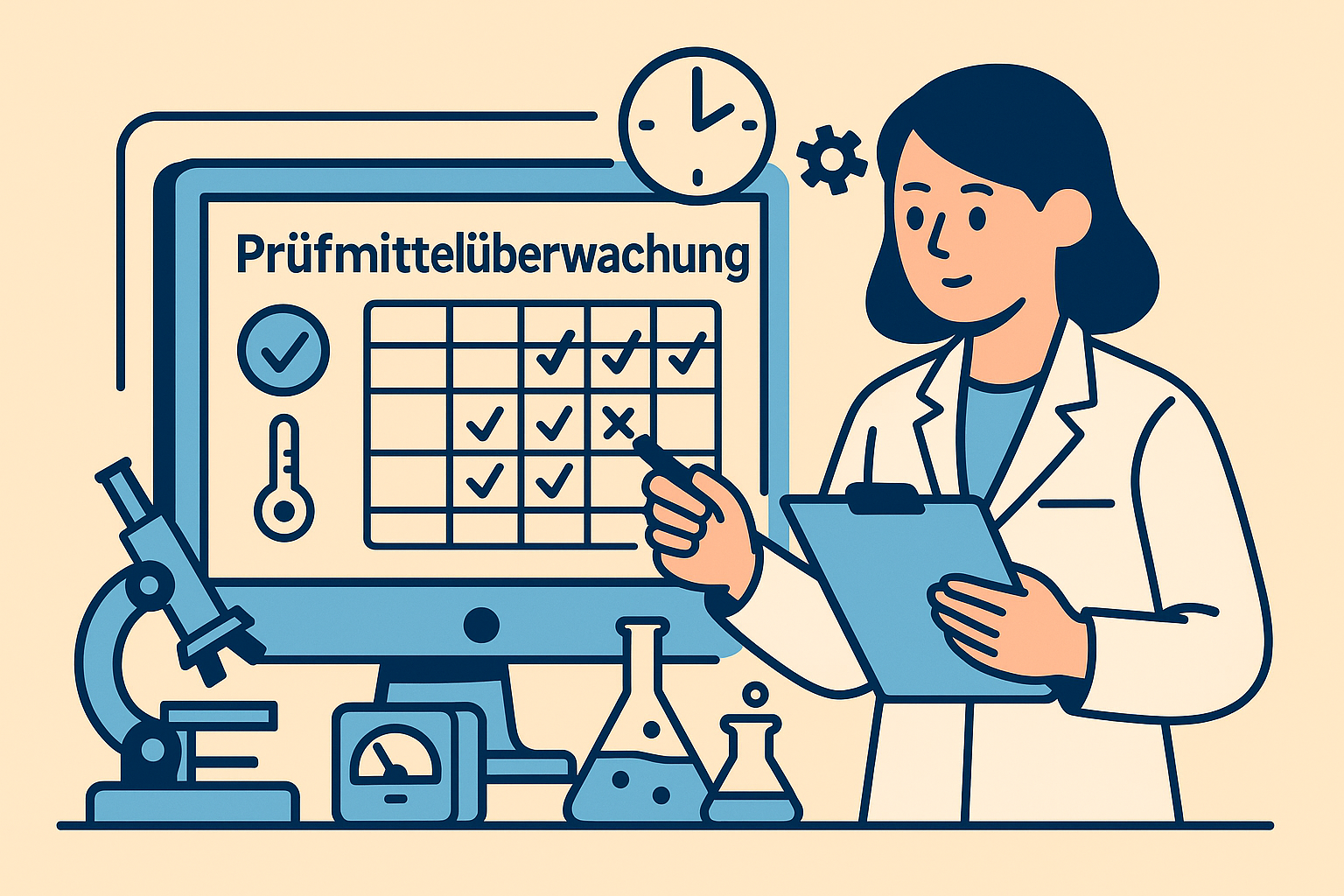
Integrated test equipment management
Automatic calibration monitoring with deadline reminders, central documentation of the test equipment and measuring equipment history and complete traceability meet the requirements of DIN EN ISO 17025, ISO 15189 and GLP Equipment Management.
Predictive maintenance
By using trends from logbooks, sensor values and frequency of use, unplanned downtimes are minimized and the availability of the devices is optimized.
Thunder AI for technical troubleshooting
The AI component analyzes error patterns, logbook entries and maintenance histories, suggests probable causes, refers to relevant passages from manuals and supports root cause analysis.
Why LabThunder replaces Excel
- Complete audit trail instead of manual versioning
- No media breaks between different systems
- Normative mapping of all regulatory requirements
- Real-time equipment status for all stakeholders
- Automated fault and maintenance detection
LabThunder enables structured fault management that supports both smaller laboratories and complex GxP environments in compliance with standards.
FAQ on error management in the laboratory
What are typical equipment faults in laboratories?
Pump errors, drift, sensor instability, temperature deviations, calibration errors, software errors, operating errors, documentation gaps and incomplete logbooks.
What is the difference between measuring equipment monitoring and test equipment management?
Measuring equipment monitoring focuses on calibration and verification. Test equipment management also includes maintenance, qualification, history, documentation and deployment planning.
What role does the ALCOA principle play in device faults?
It defines requirements for data integrity: Attributable, Legible, Contemporaneous, Original, Accurate. Error documentation must be complete, traceable and tamper-proof.
Why are Excel lists no longer sufficient for test equipment monitoring?
Excel offers no audit trails, no user management, no automation, no link to logbooks or maintenance cycles and is prone to errors. It is not sufficient for ISO 17025, ISO 15189 and GMP.
How does LabThunder specifically help with error management?
Through digital logbooks, AI-supported fault analysis, automated test equipment monitoring, predictive maintenance, standard-compliant documentation and real-time equipment status.


LabThunder:
✅ Compliant with ISO 17025, GMP/GLP, and ISO 15189
✅ Digital logbooks instead of paper chaos
✅ Thunder AI central intelligence for errors & questions
✅ Smart & predictive maintenance prevents downtime
✅ Greater independence from external service providers
✅ Up to 50% fewer service calls
✅ Easy to use - no IT required
Contact us today for a free demo:
%20new.png)













.jpg)
.jpg)

